
Landscaping Inspiration
Discover creative landscaping ideas that incorporate flowering and fruiting plants, creating a harmonious and colorful outdoor space.

Community Connection
Connect with fellow plant enthusiasts, share your experiences, and learn from others through our vibrant online community.

Environmental Awareness
Learn about the environmental importance of these plants, their role in supporting pollinators, and their contribution to sustainable ecosystems.
Discover the Botanical Wonders
At Women’s Center Flowering, we invite you to step into the enchanting world of plants that grace our planet with their vibrant blooms and delectable fruits.


Talk to us
Connect with fellow plant enthusiasts in our community forum. Share your experiences, ask questions, and learn from others who share your passion for plants.


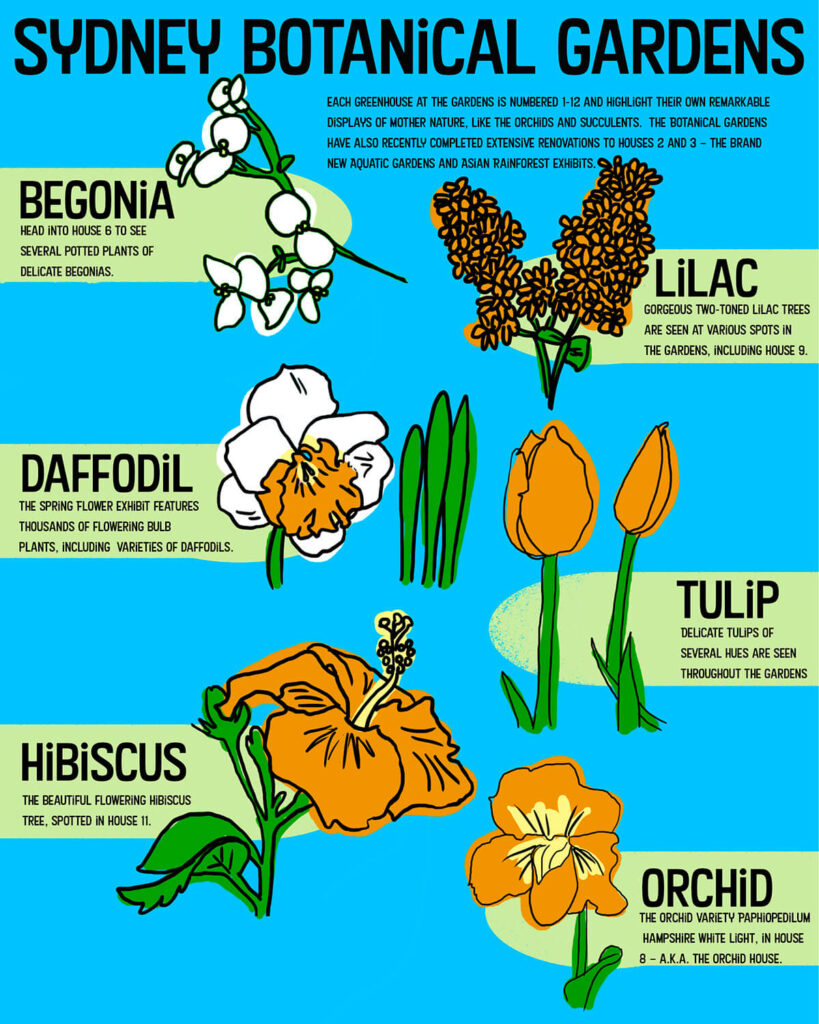
News and Articles
Dive into our articles, connect with our community at treesdownunder.com.au, and explore the incredible world of plants that bear flowers and fruits.
Why Professional Tree Removal is Crucial for Property Safety
Whispers of Love: Secret Meanings Behind Mom’s Favourite Flowers this Mothers Day
Tree Removal Services Performed by Experts
The Comprehensive Guide to Safe and Effective Tree Removal Techniques
Testimonials

Join the Women's Center Flowering Community
Join us on this botanical journey and unlock the secrets of nature’s beauty.




